Pipeline
Developing Novel Therapies
Centrexion Therapeutics recognizes the needs of the those living with chronic pain and autoimmune disease and aims to develop new, safe and effective therapies that overcome the limitations and challenges of current treatments.
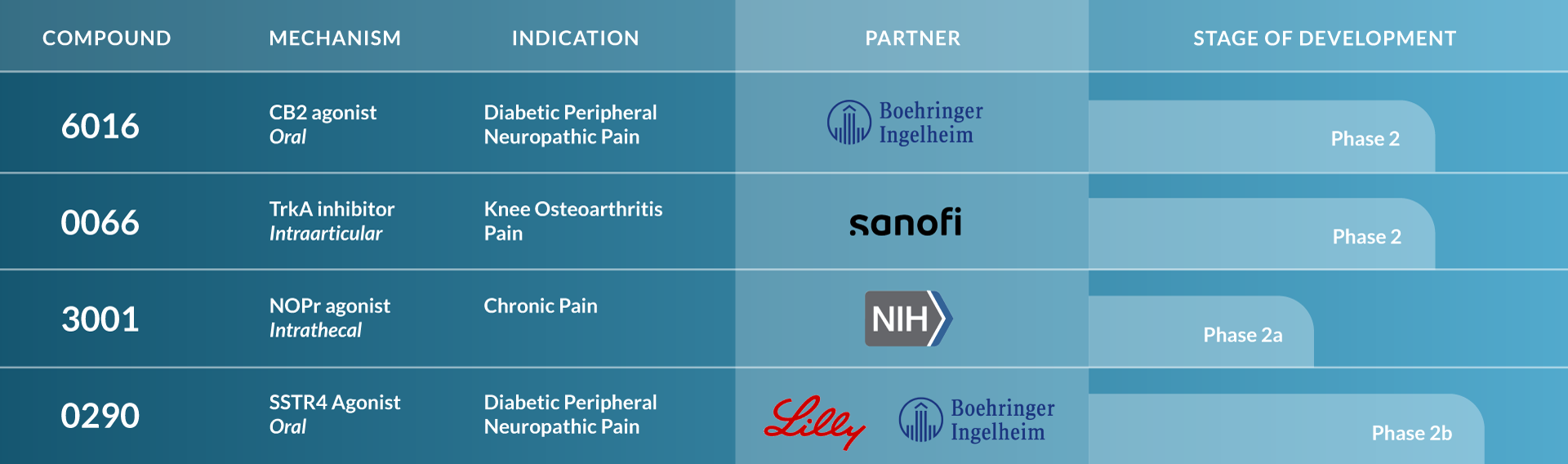
Centrexion Therapeutics Pain Pipeline
We have a broad and diversified pipeline of novel, non-opioid clinical stage medicines for the treatment of chronic pain. With our deep understanding of the underlying pathophysiology of chronic pain, we have developed a pipeline of drug candidates that are specific to both the type and source of pain. Our focus is to ensure that patients can experience relief from pain without the limitations of current treatments. We are committed to finding solutions for this highly complex and often misunderstood medical condition.
Expanding Into Immunology
We are actively exploring a variety of targets in immunology and inflammation, with a focus on differentiated medicines in indications with the highest unmet needs. Our clinical team, including our chief medical officer, Randall Stevens, M.D., have an impressive track record of success in autoimmune drug development, including the development of blockbuster and transformative therapies. Our team is well positioned to harness the industry’s momentum and excitement in immunology.
