VICTORY-1
The VICTORY-1 Phase 3 double-blind, placebo-controlled trial of CNTX-4975 in patients with moderate-to-severe knee OA commenced in February 2018. The trial has completed enrollment and is no longer recruiting patients.
Our lead product candidate, CNTX-4975, is being evaluated for the treatment of moderate to severe pain of the knee associated with osteoarthritis (OA). CNTX-4975 was granted Fast Track designation for the treatment of moderate to severe pain associated with knee OA from the U.S. FDA in January 2018.
OA occurs when the protective cartilage on the ends of the bones wears down over time, and the bones around the joints harden and form edges. These changes cause pain, swelling and difficulties moving the joint. OA also causes an inflammatory process to occur in the affected joint, further damaging the cartilage. Despite currently-available therapies that temporarily address OA pain, many patients must ultimately resort to partial or total joint replacement to address this painful condition.
We have focused on leveraging the pain-relieving properties of capsaicin – a naturally-occurring compound found in chili peppers that has been safely used as a spice and medicine for over two hundred years. More specifically, capsaicin has been used as an over-the-counter topical analgesic to provide temporary relief from mild-to-moderate pain associated with common conditions including OA and neuropathic pain.
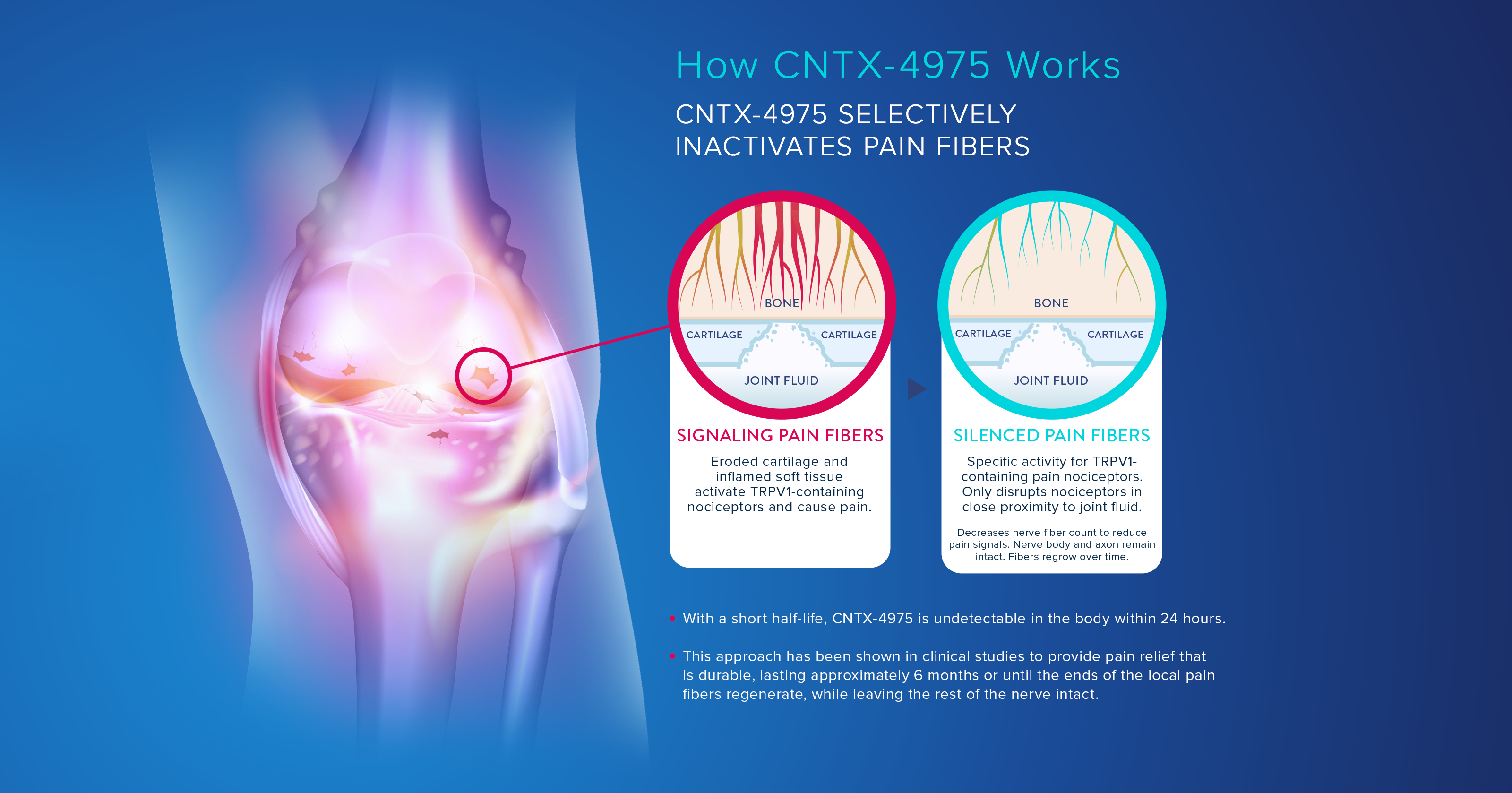
CNTX-4975 is an ultra-pure, synthetic form of trans-capsaicin that is injected directly into the site of pain. It harnesses the natural analgesic power of capsaicin to develop a proprietary injectable therapy designed to provide fast-acting, durable and targeted pain relief.
CNTX-4975 targets the TRPV1 receptor that selectively inactivates the local pain fibers transmitting pain signals to the brain, potentially providing relief that can last for up to six months, until the local pain fibers regenerate. Through its targeted delivery and highly-selective method of action, CNTX-4975 manages pain without disrupting other nerve functions.

The VICTORY-1 Phase 3 double-blind, placebo-controlled trial of CNTX-4975 in patients with moderate-to-severe knee OA commenced in February 2018. The trial has completed enrollment and is no longer recruiting patients.

The VICTORY-2 study is a 52-week clinical trial of CNTX-4975 examining capsaicin and its potential to provide extended relief from osteoarthritis knee pain. The trial has completed enrollment and is no longer recruiting patients.

The Victory-3 study is an open-label trial treating patients with knee osteoarthritis will explore different ways to administer CNTX-4975 and may explore treatment of bilateral osteoarthritis of the knees as well. The trial commenced in the second half of 2018.

Results from our TRIUMPH Phase 2b trial have been recently presented at several leading medical conferences. TRIUMPH was a randomized, double-blind, placebo-controlled, multicenter clinical trial, in which patients with chronic moderate to severe pain due to knee osteoarthritis were given a single intra-articular (into the joint) injection of CNTX-4975. The trial showed that the majority of patients receiving a 1.0 mg dose of CNTX-4975 experienced a reduction in baseline pain that occurred within two days of treatment, was statistically significant after one week and lasted at least six months. Twenty percent of patients treated with a 1.0 mg dose of CNTX-4975 experienced a 90 percent or greater reduction in pain and 61 percent achieved at least a 50 percent or greater reduction in pain compared with baseline.